After a target is selected and drug modality is chosen, the drug discovery process aims to generate and characterize a therapeutic candidate molecule, whose efficacy and safety will be investigated in clinical trials. For example, if antibody is selected as the drug modality for a receptor protein expressed on the cell surface, a candidate antibody would be generated in a process called antibody discovery.
Antibody discovery focuses on identifying and developing antibodies that can be used for therapeutic, diagnostic, and research applications. Antibodies are proteins produced by the immune system in response to foreign substances (antigens) such as viruses, bacteria, and other pathogens. Due to their high specificity and ability to bind to specific targets, antibodies have become indispensable tools in medicine and biology and the major modality of biologic drugs.
Key Steps in Antibody Discovery:
Immunization: Animals, typically mice, rabbits, or llamas, are immunized with an antigen to elicit an immune response. The choice of animal can depend on the type of antibody required (e.g., monoclonal, polyclonal, or single-domain antibodies).
Screening and Selection: After immunization, B cells (a type of white blood cell) that produce antibodies against the antigen are identified and isolated. This can be done using various techniques, including hybridoma technology for monoclonal antibodies, where B cells are fused with myeloma cells to create immortal cell lines that produce a single type of antibody.
Niels K. Jerne, Georges J.F. Köhler and César Milstein were jointly awarded the Nobel Prize in Physiology or Medicine in 1984 for theories concerning "the specificity in development and control of the immune system”and the discovery of "the principle for production of monoclonal antibodies”(hybridoma technology). In 2023, the worldwide revenue of therapeutic antibodies reached a total of 232.8 billion USD, and the majority of therapeutic antibodies have been discovered using the hydridoma technology (see how it works in the Figure at the end).
Phage Display: An alternative to animal immunization, phage display is a laboratory technique where a library of antibodies is displayed on the surface of bacteriophages (viruses that infect bacteria). The library is then exposed to the antigen, and phages that bind to the antigen are selected. This method allows for the screening of a vast number of antibody variants and does not require animal immunization.
One-half of the 2018 Nobel Prize in Chemistry was awarded jointly to George P. Smith and Sir Gregory P. Winter "for the phage display of peptides and antibodies".
Single B cell technology: it allows for the identification and characterization of antibodies from individual B cells. This technology has become increasingly important for discovering highly specific and potent antibodies. Single B cell technology enables the direct analysis of the antibody repertoires generated by an organism's immune system in response to antigens, bypassing some of the limitations associated with traditional methods like hybridoma technology or phage display.
Characterization and Optimization: Selected antibodies are further characterized for their binding affinity, specificity, and ability to elicit the desired effect (e.g., neutralizing a virus). Antibodies may also be optimized for better stability, solubility, or reduced immunogenicity.
Humanization: Many therapeutic antibodies are initially derived from mice or other animals. To reduce the risk of immune reactions when these antibodies are used in humans, they are often "humanized" by replacing most of the animal antibody sequence with human sequences, while retaining the antigen-binding region.
Production and Purification: Once an antibody has been selected and optimized, it is produced in large quantities using bioreactors and purified for use as a therapeutic agent, diagnostic tool, or research reagent.
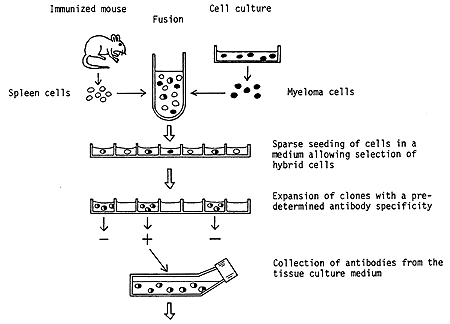
Figure showing principle steps in the production of a hybridoma. Spleen cells are prepared from animals, usually mice, which have been immunized with a selected antigen. These cells are then fused with myeloma cells maintained in culture in the laboratory. The product of this fusion is referred to as a hybridoma. Surprisingly, a hybrid of two cells can survive and also continue to divide. In this particular hybrid the myeloma cells contribute the capacity for survival, whereas the spleen cells direct the synthesis of antibodies with the preselected specificity. By special arrangements it is possible to achieve a multiplication of hybridoma cells but not of isolated myeloma cells. The hybrids obtained are propagated in a highly diluted state so that colonies deriving from single hybrid cells can be isolated. By use of a sensitive method the clones which produce the specific antibodies are identified. A particular hybridoma can then be used for future, unlimited production of a highly specific antibody.




